Abstract
BACKGROUND
Population screening of asymptomatic persons with Epstein–Barr virus (EBV) DNA or antibodies has improved the diagnosis of nasopharyngeal carcinoma and survival among affected persons. However, the positive predictive value of current screening strategies is unsatisfactory even in areas where nasopharyngeal carcinoma is endemic.
METHODS
We designed a peptide library representing highly ranked B-cell epitopes of EBV coding sequences to identify novel serologic biomarkers for nasopharyngeal carcinoma. After a retrospective case–control study, the performance of the novel biomarker anti–BNLF2b total antibody (P85-Ab) was validated through a large-scale prospective screening program and compared with that of the standard two-antibody–based screening method (EBV nuclear antigen 1 [EBNA1]–IgA and EBV-specific viral capsid antigen [VCA]–IgA).
RESULTS
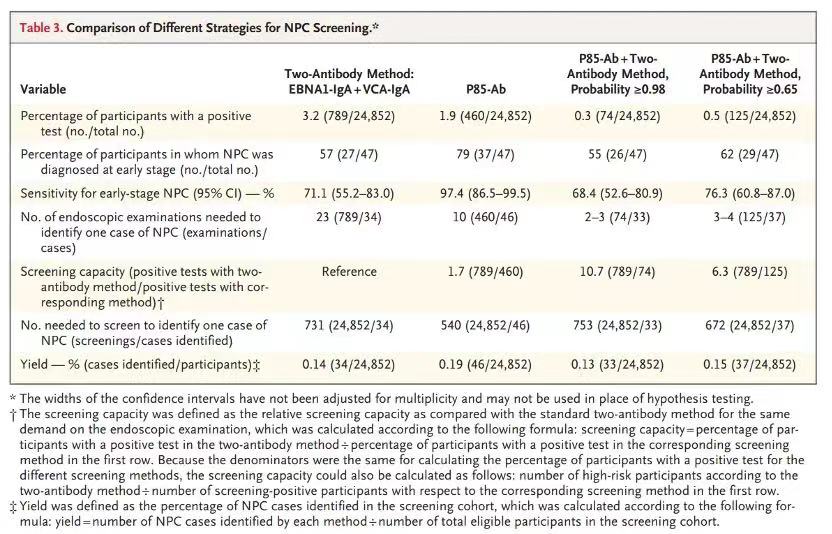
P85-Ab was the most promising biomarker for nasopharyngeal carcinoma screening, with high sensitivity (94.4%; 95% confidence interval [CI], 86.4 to 97.8) and specificity (99.6%; 95% CI, 97.8 to 99.9) in the retrospective case–control study. Among the 24,852 eligible participants in the prospective cohort, 47 cases of nasopharyngeal carcinoma (38 at an early stage) were identified. P85-Ab showed higher sensitivity than the two-antibody method (97.9% vs. 72.3%; ratio, 1.4 [95% CI, 1.1 to 1.6]), higher specificity (98.3% vs. 97.0%; ratio, 1.01 [95% CI, 1.01 to 1.02]), and a higher positive predictive value (10.0% vs. 4.3%; ratio, 2.3 [95% CI, 1.8 to 2.8]). The combination of P85-Ab and the two-antibody method markedly increased the positive predictive value to 44.6% (95% CI, 33.8 to 55.9), with sensitivity of 70.2% (95% CI, 56.0 to 81.4).
CONCLUSIONS
Our results suggest that P85-Ab is a promising novel biomarker for nasopharyngeal carcinoma screening, with higher sensitivity, specificity, and positive predictive value than the standard two-antibody method. (Funded by the National Key Research and Development Program of China and others; ClinicalTrials.gov number, NCT04085900. opens in new tab.)
Link :https://www.nejm.org/doi/pdf/10.1056/NEJMoa2301496